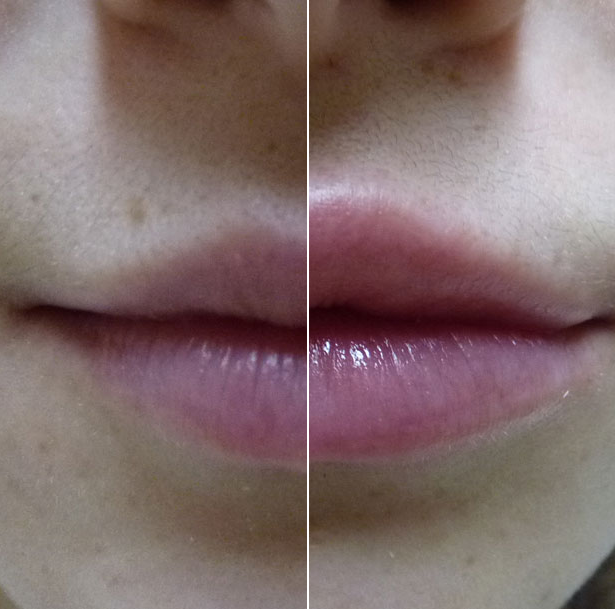
Before and after Poland syndrome
23 October 2025
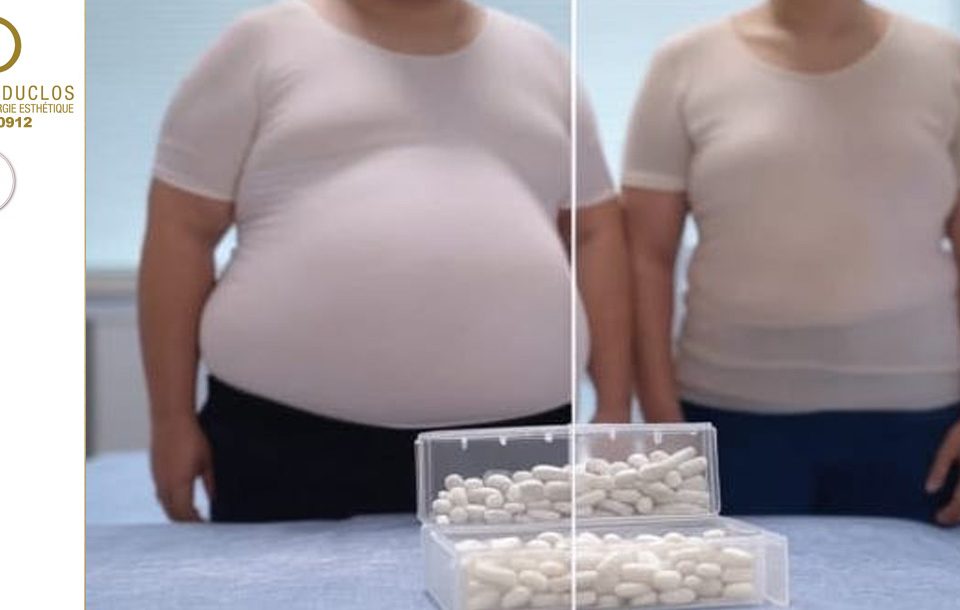
The Impact of Semaglutide on Aesthetic Surgery and Weight Loss: A Medical Revolution
1 January 2026Canada’s position on semaglutide, including its marketed forms Ozempic and Wegovy, is defined by the approvals and regulations of Health Canada, the federal agency responsible for evaluating and authorizing drugs. Here is a detailed overview of this position as of February 22, 2025, based on available information and recent developments in the use of semaglutide:
Approval and indications
Ozempic (low-dose semaglutide):
Ozempic was approved by Health Canada in 2018 for the treatment of type 2 diabetes. It is administered at a maximum dose of 1 mg per week and is intended to improve glycemic control in adults with this condition. Although weight loss is a common side effect, its use for this purpose is not officially approved in Canada and is considered an “off-label” use.
Wegovy (high-dose semaglutide):
Wegovy, which contains a higher dose of semaglutide (2.4 mg per week), was approved by Health Canada in November 2021 specifically for chronic weight management. It is indicated as an adjunct to a reduced-calorie diet and increased physical activity for adults with:
- A body mass index (BMI) of 30 kg/m² or greater (obese), or
- A BMI of 27 kg/m² or greater (overweight) with at least one weight-related comorbidity, such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.
Wegovy became available on the Canadian market in May 2024, after years of delays related to global shortages and high demand.
Regulation and Access
Public Coverage: In Canada, drug coverage varies across provinces and public and private insurance plans. Ozempic is generally reimbursed for type 2 diabetes under certain conditions (e.g., after failure of other treatments), but not for weight loss. Wegovy, as an obesity-specific treatment, is not yet widely covered by public plans, as obesity is often considered a “low priority” condition compared to other chronic diseases. Patients are therefore often required to pay out-of-pocket or rely on private insurance, which limits access due to its high cost (approximately CA$200–300 per month for Ozempic, and potentially more for Wegovy).
Shortages and Supply Management: The growing popularity of semaglutide, amplified by its use for weight loss (often publicized by influencers), has led to shortages, particularly for Ozempic. This has raised concerns about access for diabetic patients who rely on them. The Canadian government, through Health Canada and the Department of Health, is monitoring the situation to ensure that supplies are prioritized for approved indications.
Societal and Medical Position
Recognition of Obesity as a Chronic Disease: The approval of Wegovy reflects an evolution in the recognition of obesity as a serious medical condition requiring specific treatments, not just lifestyle changes. Obesity Canada, an influential organization, hailed the approval as a “paradigm shift,” noting that semaglutide offers results similar to bariatric surgery, but with less invasiveness.
Ethical and Social Challenges: The off-label use of Ozempic for weight loss has sparked debate. Some experts are concerned about overmedicalization or an excessive focus on aesthetics rather than health, while others point to the potential benefits in reducing obesity-related complications (cardiovascular disease, diabetes, etc.).
Surveillance and Safety
Health Canada continues to monitor semaglutide for side effects, including rare risks such as pancreatitis (1 in 1,000 cases per year) or gallstones (approximately 1% increased risk). Poison centre data in some provinces, such as Ontario and Quebec, have reported an increase in calls related to adverse events (nausea, accidental overdose), although these numbers remain limited and do not always distinguish between use for diabetes and weight loss.
Conclusion
Canada’s position on semaglutide is clear: it is recognized as a valuable tool for diabetes (Ozempic) and obesity management (Wegovy), with formal Health Canada approvals. However, access to semaglutide remains hampered by issues of cost, coverage, and availability, while off-label use for weight loss raises logistical and ethical concerns. The country is taking a cautious but gradual approach, integrating the drug into a broader strategy to combat obesity while ensuring that diabetic patients who need it are protected. This position could change with the arrival of new treatments or adjustments in reimbursement policies.